Vitamin C Liver Disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is recognized as the most common chronic liver disease globally, with almost 25% prevalence in the general population (1). In the early 2000s, the prevalence of NAFLD in China was 23.8% and reached 32.9% in 2018, which was responsible for over a third (37%) of the incidence of central obesity or diabetes (2, 3). Without intervention, NAFLD may progress from simple hepatic steatosis to advanced steatohepatitis, even to fibrosis, cirrhosis, and hepatocellular carcinoma (4). Therefore, identifying approaches to prevent NAFLD is one of the public health problems that need to be solved urgently.
Previous studies have reported the impact of oxidative stress and inflammation on NAFLD (5–7). Vitamin C (VC), as an effective water-soluble antioxidant, has a scavenging effect on excessive free radicals in the body and a protective effect on tissue damage caused by oxidative stress, and it probably plays a protective role against NAFLD (8, 9). The association between dietary VC intake and NAFLD has been studied, but not extensively, and with conflicting results (10–13). Han et al. revealed a significant positive association between low VC intake and NAFLD in the male population in a cross-sectional study (11). In contrast, another small sample cross-sectional study suggested that both dietary VC intake and plasma VC concentration were of similar levels between patients with NAFLD and healthy controls (12). Moreover, the clinical burden of NAFLD is not only confined to liver-related morbidity and mortality, but NAFLD is a multisystem disease affecting extrahepatic organs and regulatory pathways (14). Epidemiological studies have also shown an association between VC status and reduced insulin resistance and improved blood glucose control (15–17). Meanwhile, higher plasma VC was associated with a lower risk of developing type 2 diabetes (T2DM) (18). A previous study suggested that supplementation of the diet with kiwifruit, which is rich in VC, enhanced plasma VC status and also decreased the HbA1c levels (19). A recent meta-analysis investigating VC supplementation and insulin resistance found that doses of ≥200 mg/day VC significantly reduced glucose concentrations in patients with T2DM, mainly if the intervention was for more than 30 days and in older individuals (20).
However, the possible mechanisms of VC in improving liver health and glucose metabolism remain unclear. Adiponectin, which exerts marked insulin-sensitizing and anti-inflammatory effects (21), has been reported to be involved in the pathogenesis of non-alcoholic fatty liver, and a previous study demonstrated that a decrease in adiponectin levels is an independent risk factor of developing NAFLD (22, 23). Interestingly, our previous study and other study showed that VC treatment could increase the secretion of high molecular weight (HMW) adiponectin from human hepatocytes and adipocytes (24, 25). In addition to having lower levels of adiponectin, patients with NAFLD also have increased intestinal permeability and the changes in intestinal microbiota may also be the driving force for the progress of NAFLD (26–29). However, the effect of VC on the intestinal microbiota is unclear.
By now, no studies have been performed to determine whether VC alone has a causal effect on liver function and glucose homeostasis parameters in patients with NAFLD. Therefore, in this double-blind, randomized controlled trial (RCT), we aimed to find out whether VC supplementation could improve liver function and other associated metabolic markers in NAFLD individuals and explored possible mechanisms.
Patients and Methods
Patients
From March to May 2020, patients with NAFLD aged 18–60 years old and who have lived in Xianyang for more than 10 years were recruited from the Medical Examination Center of Xianyang Central Hospital, Xi'an, Shaanxi, China. Patients who were newly diagnosed with NAFLD were included (30). Patients affected by hepatitis B and C, and those with cardiac, renal, hyperuricemia, hyperoxaluria, hemochromatosis, autoimmune, cirrhosis, and other metabolic diseases were excluded. Exclusion criteria also included insulin treatment, smoking habits, alcohol intake (>20 g/day), other antioxidant supplements or medications taking in the past 3 months, recreational or statins drug use, and exposure to environmental toxins known to induce liver steatosis. According to the sample size calculation formula, the number of cases required for each group was 25. Taking a dropout rate of 20% into consideration, at least 90 volunteers will be recruited. Overall, 98 participants were enrolled in the study. This trial was registered at ClinicalTrials.gov (http://www.chictr.org.cn); Identifier: ChiCTR2000033171. The study was consistent with the ethical guidelines of the Helsinki 1975 Declaration and was approved by the Ethics Committee of Xi'an Jiaotong University and Xianyang Central Hospital. Signed informed consents were obtained from all the participants, and all of them were told that there is no need to change eating habits except for the intervention.
Study Supplements and Allocation
Participants were assigned to one of three different treatment groups using stratified randomization. Randomization was stratified by gender, age, weight, body mass index (BMI), and waist circumference. Based on the Chinese Residents' Dietary Guidelines, the proposed intake for chronic non-communicable diseases (PI-NCD) is 200 mg/day for adults (31). Since it was tough to produce a placebo with the same taste as VC (Blackmores, Australia), the Low (dose) group took one tablet of VC (250 mg/day) per day, and the High group was supplemented with 2,000 mg/day, which is the tolerable upper intake level. The Medium group was also designed and assigned to 1,000 mg/day of VC. Participants were asked to take or chew the supplements before meals. During the 12 weeks intervention time, they were also asked to visit the intervention staff every 2 weeks where their clinical symptoms and side effects from VC supplementation were assessed, compliance was checked based on the number of unconsumed tablets, and tablets for further use were dispensed. In addition, subjects were instructed to maintain the same lifestyle and diet during the trial as usual.
Procedures
Participants were asked to provide demographic information and completed self-administered diet and physical activity questionnaires before the intervention. Anthropometric parameters (body weight, height, and waist and hip circumferences) and systolic and diastolic blood pressures were assessed according to standard methods. BMI was calculated as body weight divided by height squared (kg/m2). Fasting blood was collected at baseline and week 12 by an antecubital venous puncture. Serum or plasma samples were centrifuged at 1,500 g for 15 min at 4°C and frozen at −80°C. Plasma VC content was detected by 1,290 Infinity Ultrahigh-Performance Liquid Chromatography (UHPLC) from Agilent Company in Guangzhou KingMed Center for Clinical Laboratory Co., Ltd., Guangdong Province, China. The detection method has been described in detail previously (32). Briefly, the samples were pretreated by protein precipitation, centrifugation, etc. Then, the processed samples were introduced into the UHPLC system with the mobile phase and then chromatographically separated to reach the diode array detector. Finally, the VC concentration was calculated by the strength of the signal response. All other blood indicators associated with the liver function such as total protein (TP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (γ-GT), alkaline phosphatase (ALP), total bilirubin (TBIL), serum indirect bilirubin (IBIL), serum direct bilirubin (DBIL), total cholesterol (TC), and triglyceride (TG) were tested twice in parallel using Hitachi 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan) to obtain the average value. Plasma human total and HMW adiponectin were measured using ELISA test kits purchased from Abcam in the United Kingdom (Cat. # ab99968) and MERCK, Germany (Cat. # EZHMWAN-65K), respectively. Finally, the absorbance (Optical Delnsity, OD value) was measured at a wavelength of 450 nm with a microplate reader to calculate the concentration.
We also collected the feces of volunteers from the Low and Medium groups at baseline and week 12 for 16S rDNA Amplicon Sequencing (Novogene Company, China). Operational taxonomic units (OTUs) clustering and species classification analysis based on the clean data obtained by sequencing were performed. According to the OTU clustering results, species annotations were made on the representative sequence of each OTU, and the corresponding species information and species-based abundance distribution were obtained. At the same time, the abundance, alpha diversity calculation, Venn map, and petal map analysis of OTUs were performed to obtain the species richness and uniformity information among different samples or groups.
Statistical Analysis
Differences in baseline measurements among groups were assessed using the chi-squared test for categorical variables and variance tests for continuous measures. The one-way ANOVA test and the Student–Newman–Keuls test were used to determine the significance of the difference. In non-parametric analyses, the significance of the changes within and between groups was assessed by the Wilcoxon rank-sum test. Analyses were carried out using SPSS software (version 24) (SPSS Inc., Chicago, USA). Significance was defined as P < 0.05 (two-tailed).
Results
Baseline Characteristics
Of 98 subjects enrolled, 84 completed the study (Figure 1). A total of 14 subjects terminated early. Five withdrew due to personal reasons, four lost due to inconvenience, and five were lost to follow-up. The samples of the stool of volunteers were collected before and after the intervention. Only 12 people, 6 from the Low group and 6 from the Medium group, provided samples both before and after the intervention. The mean age of participants was 40.6 years, and 58.3% were women. The fasting glucose, lipids, and plasma VC status were comparable among the three groups at baseline (Table 1). After the intervention, plasma VC levels were significantly elevated in the Medium group (before 56.67 ± 19.27 vs. after 49.28 ± 15.82, P = 0.032) and the High group (before 64.40 ± 19.06 vs. after 50.27 ± 16.03, P = 0.007; Figure 2). Interestingly, men had lower plasma VC levels before the intervention than women (men 39.90 ± 13.73 vs. women 55.55 ± 13.64, P < 0.001). Nevertheless, after the intervention, there was a remarkable increase in the men among the three groups (P = 0.024). There was also an increase of plasma VC levels in the women before and after the intervention, but without static significance (P = 0.018; Supplementary Figure 1).
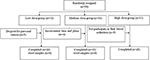
Figure 1. Flow diagram of participant progress through the study. 1means nine participants provided samples before the intervention, three participants lose after the intervention, six participants from the Low group provided samples both before and after the intervention finally. 2means seven participants provided samples before the intervention, one participant lose after the intervention, six from the Medium group provided samples both before and after the intervention finally. 3means five participants provided samples before the intervention, all participants lose after the intervention, there are no complete samples finally in the High group.

Table 1. Baseline characteristics of participantsa.
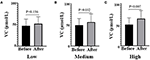
Figure 2. Plasma VC levels before and after the intervention in all three groups, respectively. (A) means Low dose group, (B) means Medium dose group, (C) means High dose group. Black means VC plasma concentration (μmol/l) before the intervention, gray means VC plasma concentration after the intervention; paired t-test used to test the difference before and after the intervention in three groups. VC, vitamin C.
Positive Modulatory Effect on Liver Function
As shown in Table 2, after the intervention, some serological indicators of the three groups all improved statistically. First, in the low group, compared with baseline, the AST, ALP, TBIL, and DBIL indexes of the Low group decreased. In the Medium group, AST, ALT, γ-GT, ALP, and DBIL indexes decreased. In the High group, the levels of γ-GT, ALP, TBIL, DBIL, and triglycerides also decreased. Second, compared among groups, the changes of all the liver function indicators were similar except for ALT and AST, whose P-values were close to 0.05. Further comparison of specific differences between groups showed that compared with the High group, the Medium group had a more significant decrease in AST [Medium, −5.00 (−10.25, −1.75) vs. High, −2.50 (−7.75, 0.00), P = 0.02]. Medium group had a more significant decrease in ALT [Medium, −8.00 (−18.00, −1.75) vs. High, −3.50 (−13.75, 4.25), P = 0.05; Medium, −8.00 (−18.00, −1.75) vs. Low, −3.00 (−9.00, 5.50), P = 0.031]. However, there was no statistically significant difference in ALT or AST between the Low and the High groups.
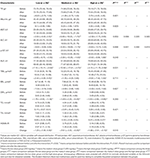
Table 2. Metabolic indicators change during the triala.
Effects on Glucose Metabolism
After the intervention, blood glucose metabolism status statistically significantly improved in all three groups (Figure 3). The blood glucose levels were significantly decreased in the Medium group (before 5.05 ± 0.51 vs. after 4.75 ± 0.53, P = 0.002) and the High group (before 5.26 ± 0.80 vs. after 4.88 ± 0.98, P = 0.004), while the insulin levels of participants in all the groups reduced obviously [Low (before 22.24 ± 12.50 vs. after 15.92 ± 10.27, P < 0.001); Medium (before 19.22 ± 14.54 vs. after 8.46 ± 7.55, P < 0.001); High (before 22.24 ± 21.1 vs. after 10.54 ± 12.45, P < 0.003)]. However, there was no difference among groups (glucose, P = 0.753; insulin, P = 0.389; data not shown). Notably, there was a remarkable decrease in homeostasis model assessment for insulin resistance (HOMA-IR) among the three groups, especially the Medium group (before 4.61 ± 3.51 vs. after 2.62 ± 2.41, P < 0.001; Table 2). Then, we also detected the HWM adiponectin and total adiponectin levels of patients and found a significant elevation after the intervention with VC in all groups [total adiponectin, Low (before 41.77 ± 33.26 vs. after 110.41 ± 74.87, P < 0.001), Medium (before 30.57 ± 17.93 vs. after 78.48 ± 50.24, P < 0.001), High (before 43.57 ± 18.76 vs. after 70.29 ± 48.25, P < 0.001); HWM adiponectin, Low (before 2.49 ± 1.73 vs. after 8.79 ± 4.69, P < 0.001), Medium (before 4.18 ± 2.54 vs. after 9.09 ± 4.31, P < 0.001), High (before 2.57 ± 3.65 vs. after 9.70 ± 5.49, P = 0.004)]. However, the elevation of both total and HMW adiponectin was not remarkable among the groups (data not shown) (Figure 4).
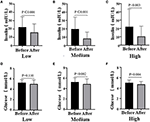
Figure 3. Fasting blood glucose levels (A, Low dose group; B, Medium dose group; C, High dose group) and insulin levels (D, Low dose group; E, Medium dose group; F, High dose group) before and after the intervention in all three groups, respectively. Black means glucose or insulin plasma concentration (mmol/l, mIU/l) before the intervention, gray means glucose or insulin plasma concentration after the intervention; paired t-test used to test the difference before and after the intervention in the three groups.
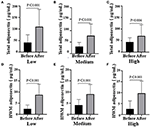
Figure 4. Total adiponectin (A, Low dose group; B, Medium dose group; C, High dose group) and HWM adiponectin levels (D, Low dose group; E, Medium dose group; F, High dose group) before and after the intervention in the three groups, respectively. Black means total adiponectin or HWM adiponectin plasma concentration (μg/ml) before the intervention, gray means total adiponectin or HWM adiponectin plasma after the intervention; paired t-test used to test the difference before and after the intervention in all three groups.
Changes in the Intestinal Microbial Community
For the 16S rRNA sequencing of the intestinal microbiota of patients with NAFLD, there were six volunteers in the Low and the Medium groups, respectively, and 24 fecal biological samples were collected. The alpha diversity analysis indicated that all the four diversity indexes (ACE, Shannon, chao1, and Simpson) in the two groups showed an upward trend (Figure 5). For species community analysis (Figure 6), compared with the control group (250 mg/day), the Bacteroides level in the middle-dose group declined, the Firmicutes rose, and the proportion of Firmicutes and Bacteroides (Firmicutes to Bacteroidetes, F/B) decreased.
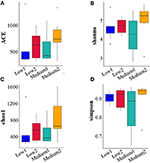
Figure 5. Analysis and comparison of multiple indexes before and after the intervention in the Low and the Medium groups. The study of microbial diversity in community ecology can reflect the abundance and diversity of microbial communities through a single sample diversity analysis [α(alpha) diversity], including a series of statistical analysis indexes to estimate the species abundance and diversity of environmental communities diversity. The picture showed the four indexes [ACE (A), shannon (B), chao1 (C), and simpson (D)] of the α diversity analysis of 6 subjects in the Low group and Medium group respectively before and after the intervention. The higher the values of the four indexes, the more abundant species in the community. Low1 means before the intervention in the low-dose group, Low2 means after the intervention in the low-dose group, Medium1 means before the intervention in the medium-dose group, and Medium2 means after the intervention in the medium-dose group.
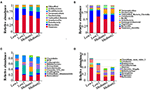
Figure 6. Analysis and comparison of relative abundance of top 10 before and after the intervention in the Low and the Medium groups at phylum, class, order, and genus level. The higher the relative proportion, the more abundance in the community at different biological classification level. (A) means phylum level; (B) means class level; (C) means order level; and (D) means genus level.
Discussion
In this study, we observed that oral VC supplementation improved liver health and glucose metabolism in patients with NAFLD. The intake of 1,000 mg/day VC showed a profound effect. Vitamin C supplementation increased plasma VC, adiponectin, HMW adiponectin levels, and the diversity of intestinal microbiota. Furthermore, decreased the majority of parameters associated with liver function, fasting glucose level, and HOMA-IR. Among the participants, men were likely to get more benefits from the supplementation.
Regarding the nutritional risk factors, excessive energy intake but relatively minor intake of micronutrients contributes more to NAFLD (33). Hence, lifestyle changes and antioxidant therapy are widely used to prevent and treat NAFLD (34). Among antioxidants, vitamin E is now widely recommended clinically at present. The largest trial by now revealed that vitamin E treatment showed a superior effect than pioglitazone on improving histological and biochemical features in subjects with non-alcoholic steatohepatitis (NASH) (35). However, vitamin E would increase insulin resistance and plasma triacylglycerols when administered for 2 years and even was associated with excess mortality in primary and secondary prevention trials (36, 37). Compared with fat-soluble vitamin E that can accumulate after long-term use, VC is water-soluble and remarkably safe, even at 10–100 times the recommended dietary allowance when taken orally (38). Some previous studies have indicated that the combined supplementation of VC and other nutrients, such as vitamin E or resveratrol, can alleviate hepatic steatosis, but the effect of VC alone on liver function was still not apparent (39, 40). Animal experiments showed that VC was involved in regulating circulating and hepatic lipids homeostasis (41). In mice, a moderate dose (30 mg/kg/day) was beneficial for the preventing and treating of NAFLD caused by a high-fat diet (42). Although the epidemiological study also supported the association between inadequate VC intake and NAFLD, there is no direct evidence that can prove the benefits of supplementing VC on patients with NAFLD (43). In our study, we found that 12 weeks of VC intervention remarkably improved the indicators of liver function in patients with NAFLD. Our results suggested that as adjuvant therapy for daily health prevention and improvement of prognosis of NAFLD, routine supplementation of VC can help the recovery of liver function.
Non-alcoholic fatty liver disease is associated with an increased risk of cardiovascular disease, dyslipidemia, and T2DM in adults (44, 45). Several hypotheses were generating to understand the metaorganism pathways that influence the development and progression of NAFLD. What has been determined is that it is strongly associated with obesity and insulin resistance (46–48). Consistently, in this study, we also observed that all the patients were overweight, and their waist circumferences were very close to the cut-off point according to the Chinese standard. Since all participants were asked to keep their dietary habits, they had rare weight changes no matter how much VC was administered. In terms of glucose homeostasis, we found the fasting plasma insulin levels were close to the upper level of normal standard, even though their fasting glucose levels were still normal. It appeared likely that those patients had a high risk of getting the possibility to progress to T2DM soon, which was consistent with the epidemiological fact that patients with NAFLD are more likely to develop other metabolic diseases, especially T2DM (49). Interestingly, our results showed that 12 weeks of VC supplementation improved the glucose metabolism of patients. Specifically, fasting plasma insulin and glucose contents (except for the Low group) were all reduced after the intervention markedly, accompanied by a lower degree of insulin resistance. Similarly, the most favorable effect was still seen in the Medium group. Although it was hard to tell the causal relationship between the recovery of liver function and improved glucose metabolism, our results at least indicated that the intervention of VC would decelerate the disease progression of patients with NAFLD.
Previous studies suggested that adiponectin, which has many favorable effects on metabolic diseases, may be involved in the pathogenesis of NAFLD (22, 23). Our previous study showed that VC pretreatment might be a new treatment method of NAFLD/NASH through attenuate hepatocyte stress induced by TNFα via activation of the FGF21/FGFR2/adiponectin pathway (24). Expectedly, in this study, after the intervention, even at the smallest dose (250 mg/day), total adiponectin and HWM adiponectin levels showed a significant upward trend, which may contribute to the improvement of both liver function and glucose metabolism in patients with NAFLD. To the best of our knowledge, this is the first time to report the effect of VC supplementation on adiponectin production in human individuals, which is of great significance to clarify the protective role of VC in NAFLD. More interestingly, we found that the men seemed to get more benefits from VC supplementation than the women. This finding may be explained by the truth that men generally consume fewer fresh vegetables and fruits daily than the women at baseline (data not shown), and their initial VC levels were lower. Our findings were consistent with a recent study that showed that the inverse association between dietary VC intake and NAFLD in middle-aged and older adults was stronger in the male population (10).
Gut microbiota and gut-liver axis dysfunction are critical for NAFLD progression and the development of more severe inflammatory and fibrotic stages NASH (50). Several studies have suggested that in addition to changes in adiponectin, changes in the intestinal microbiota may also be the driving force for the progress of NAFLD (28, 29). Our research also found that taking 250 mg/day and 1,000 mg/day of VC daily for 12 weeks can improve the intestinal microbiota of patients with NAFLD by increasing the diversity of intestinal microbiota and the relative proportion of beneficial bacteria. Bacteroidetes and Firmicutes are the dominant "good" bacteria and "bad" bacteria, respectively. In the human intestinal tract, a person with NAFLD showed a higher Firmicutes to Bacteroidetes (F/B) ratio than a healthy population (51, 52). In this study, we found that only 1,000 mg/day of VC supplementation for 12 weeks increased the diversity of species and the dominant microbiota. Unfortunately, only 12 people from the Low and Medium groups provided stool samples. Although the sample size was small, our results hint that VC supplementation may produce beneficial effects on patients with NAFLD by affecting the intestinal microbiota, which may, in turn, promote liver function and other related metabolic parameters in patients with NAFLD through the gut-liver axis.
The main strength of our study was the randomized controlled design and stratification by age, gender, and BMI, which eliminates interindividual differences. Furthermore, our research filled the gap in the trial of VC intervention alone in patients with NAFLD and revealed the beneficial effects. However, there were also some limitations in our research. First, there was no placebo control in our study because it was challenging to make a placebo with the same odor and color as VC. Regardless, it was also appropriate to take a dose that is close to the PI-NCD of VC (200 mg/day) to meet the favorable principles in ethics. Second, due to the relatively small group of participants, the differences in couples of indicators regarding liver function were not significantly different though a good effect was present. To obtain more generalizable conclusions, more extensive population studies are still needed.
In conclusion, to our best knowledge, this is the first study that determined the effect of oral VC supplementation on patients with NAFLD. Daily supplementation with VC, especially 1,000 mg/day, can help promote liver function recovery and glucose homeostasis in patients with NAFLD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xi'an Jiaotong University and Xianyang Central Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XLu, ZH, and XLi designed the research. ZH, HY, SW, ZX, PW, XG, WZ, and JG conducted the research. ZH, XLi, and HY analyzed the data and performed the statistical analysis. XLi, ZH, and XLu wrote the paper and had primary responsibility for the final content. All the authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81874263).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.745609/full#supplementary-material
Abbreviations
NAFLD, nonalcoholic fatty liver disease; VC, vitamin C; RCT, randomized controlled trial; HMW, high molecular weight; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; ALP, alkaline phosphatase; TBIL, total bilirubin; DBIL, direct bilirubin; HOMA-IR, homeostasis model assessment for insulin resistance; T2DM, type 2 diabetes; PI-NCD, proposed intake for chronic non-communicable disease; UHPLC, ultrahigh performance liquid chromatography; OTUs, operational taxonomic units; TG, triglycerides; NASH, nonalcoholic steatohepatitis.
References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. (2006) 40:930–5. doi: 10.1097/01.mcg.0000212608.59090.08
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Ferro D, Baratta F, Pastori D, Cocomello N, Colantoni A, Angelico F, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: gut-derived lipopolysaccharides and oxidative stress. Nutrients. (2020) 12:2762. doi: 10.3390/nu12092762
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroentero. (2014) 20:14205–18. doi: 10.3748/wjg.v20.i39.14205
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Padayatty SJ, Katz A, Wang YH, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. (2003) 22:18–35. doi: 10.1080/07315724.2003.10719272
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Tamari Y, Nawata H, Inoue E, Yoshimura A, Yoshii H, Kashino G, et al. Protective roles of ascorbic acid in oxidative stress induced by depletion of superoxide dismutase in vertebrate cells. Free Radical Res. (2013) 47:1–7. doi: 10.3109/10715762.2012.734916
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Wei J, Lei GH, Fu L, Zeng C, Yang T, Peng SF. Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS ONE. (2016) 11:e0147985. doi: 10.1371/journal.pone.0147985
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Han JM, Jo AN, Lee SM, Bae HS, Jun DW, Cho YK, et al. Associations between intakes of individual nutrients or whole food groups and non-alcoholic fatty liver disease among Korean adults. J Gastroen Hepatol. (2014) 29:1265–72. doi: 10.1111/jgh.12520
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Da Silva HE, Arendt BM, Noureldin SA, Therapondos G, Guindi M, Allard JP. A cross-sectional study assessing dietary intake and physical activity in Canadian patients with nonalcoholic fatty liver disease vs healthy controls. J Acad Nutr Diet. (2014) 114:1181–94. doi: 10.1016/j.jand.2014.01.009
PubMed Abstract | CrossRef Full Text | Google Scholar
13. Chan R, Wong VW, Chu WC, Wong GL, Li LS, Leung J, et al. Diet-Quality scores and prevalence of nonalcoholic fatty liver disease: a population study using proton-magnetic resonance spectroscopy. PLoS ONE. (2015) 10:e0139310. doi: 10.1371/journal.pone.0139310
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Hirashima O, Kawano H, Motoyama T, Hirai N, Ohgushi M, Kugiyama K, et al. Improvement of endothelial function and insulin sensitivity with vitamin C in patients with coronary spastic angina: possible role of reactive oxygen species. J Am Coll Cardiol. (2000) 35:1860–6. doi: 10.1016/S0735-1097(00)00616-1
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Park S, Ham JO, Lee BK. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition. (2015) 31:111–8. doi: 10.1016/j.nut.2014.05.011
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Zhou CL, Na LX, Shan RQ, Cheng Y, Li Y, Wu XY, et al. Dietary vitamin C intake reduces the risk of type 2 diabetes in Chinese adults: HOMA-IR and T-AOC as potential mediators. PLoS ONE. (2016) 11:e0163571. doi: 10.1371/journal.pone.0163571
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Namkhah Z, Ashtary-Larky D, Naeini F, Clark CCT, Asbaghi O. Does vitamin C supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? A systematic review and dose-response meta-analysis. Pharmacol Res. (2021) 169:105665. doi: 10.1016/j.phrs.2021.105665
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Wilson R, Willis J, Gearry RB, Hughes A, Lawley B, Skidmore P, et al. SunGold kiwifruit supplementation of individuals with prediabetes alters gut microbiota and improves vitamin c status, anthropometric and clinical markers. Nutrients. (2018) 10:895. doi: 10.3390/nu10070895
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Ashor AW, Werner AD, Lara J, Willis ND, Mathers JC, Siervo M. Effects of vitamin C supplementation on glycaemic control: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. (2017) 71:1371–80. doi: 10.1038/ejcn.2017.24
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Trujillo ME, Scherer PE. Adiponectin–journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. (2005) 257:167–75.
PubMed Abstract | Google Scholar
22. Mirza MS. Obesity, visceral fat, and NAFLD: querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterol. (2011) 2011:592404. doi: 10.5402/2011/592404
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroentero. (2013) 19:802–12. doi: 10.3748/wjg.v19.i6.802
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Gu X, Luo X, Wang Y, He Z, Li X, Wu K, et al. Ascorbic acid attenuates cell stress by activating the fibroblast growth factor 21/fibroblast growth factor receptor 2/adiponectin pathway in HepG2 cells. Mol Med Rep. (2019) 20:2450–8. doi: 10.3892/mmr.2019.10457
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Rose FJ, Webster J, Barry JB, Phillips LK, Richards AA, Whitehead JP. Synergistic effects of ascorbic acid and thiazolidinedione on secretion of high molecular weight adiponectin from human adipocytes. Diabetes Obes Metab. (2010) 12:1084–9. doi: 10.1111/j.1463-1326.2010.01297.x
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. (2009) 49:1877–87. doi: 10.1002/hep.22848
PubMed Abstract | CrossRef Full Text | Google Scholar
27. Doulberis M, Kotronis G, Gialamprinou D, Kountouras J, Katsinelos P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism. (2017) 71:182–97. doi: 10.1016/j.metabol.2017.03.013
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. (2016) 63:764–75. doi: 10.1002/hep.28356
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Gao X, Fan JG Study Group of Liver and Metabolism Chinese Society of Endocrinology. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: consensus statement from the study group of liver and metabolism, Chinese society of endocrinology. J Diabetes. (2013) 5:406–15. doi: 10.1111/1753-0407.12056
PubMed Abstract | CrossRef Full Text
32. Iglesias J, Gonzalez MJ, Medina I. Determination of ascorbic and dehydroascorbic acid in lean and fatty fish species by high-performance liquid chromatography with fluorometric detection. Eur Food Res Technol. (2006) 223:781–6. doi: 10.1007/s00217-006-0269-1
CrossRef Full Text | Google Scholar
33. Chakravarthy MV, Waddell T, Banerjee R, Guess N. Nutrition and nonalcoholic fatty liver disease: current perspectives. Gastroenterol Clin North Am. (2020) 49:63–94. doi: 10.1016/j.gtc.2019.09.003
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. (2010) 362:1675–85. doi: 10.1056/NEJMoa0907929
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. (2012) 55:885–904. doi: 10.1007/s00125-011-2446-4
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. (2007) 297:842–57. doi: 10.1001/jama.297.8.842
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Izdebska M, Piatkowska-Chmiel I, Korolczuk A, Herbet M, Gawronska-Grzywacz M, Gieroba R, et al. The beneficial effects of resveratrol on steatosis and mitochondrial oxidative stress in HepG2 cells. Can J Physiol Pharm. (2017) 95:1442–53. doi: 10.1139/cjpp-2016-0561
PubMed Abstract | CrossRef Full Text | Google Scholar
40. Ivancovsky-Wajcman D, Fliss-Isakov N, Salomone F, Webb M, Shibolet O, Kariv R, et al. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Digest Liver Dis. (2019) 51:1698–705. doi: 10.1016/j.dld.2019.06.005
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Takahashi K, Kishimoto Y, Konishi T, Fujita Y, Ito M, Shimokado K, et al. Ascorbic acid deficiency affects genes for oxidation-reduction and lipid metabolism in livers from SMP30/GNL knockout mice. Bba-Gen Subjects. (2014) 1840:2289–98. doi: 10.1016/j.bbagen.2014.03.019
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Zeng Q, Zhao L, Meng C, Zhao X, Liu Y, Shi R, et al. Prophylactic and therapeutic effects of different doses of vitamin C on high-fat-diet-induced non-alcoholic fatty liver disease in mice. Biomed Pharmacother. (2020) 131:110792. doi: 10.1016/j.biopha.2020.110792
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. (2017) 66:1138–53. doi: 10.1136/gutjnl-2017-313884
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. (2003) 37:917–23. doi: 10.1053/jhep.2003.50161
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. (2013) 59:138–43. doi: 10.1016/j.jhep.2013.02.012
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Smith GI, Shankaran M, Yoshino M, Schweitzer GG, Chondronikola M, Beals JW, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. (2020) 130:1453–60. doi: 10.1172/JCI134165
PubMed Abstract | CrossRef Full Text | Google Scholar
51. Monga Kravetz A, Testerman T, Galuppo B, Graf J, Pierpont B, Siebel S, et al. Effect of gut microbiota and PNPLA3 rs738409 variant on nonalcoholic fatty liver disease (NAFLD) in obese youth. J Clin Endocrinol Metab. (2020) 105:dgaa382. doi: 10.1210/clinem/dgaa382
PubMed Abstract | CrossRef Full Text | Google Scholar
52. Jasirwan COM, Muradi A, Hasan I, Simadibrata M, Rinaldi I. Correlation of gut firmicutes/bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci Microbiota Food Health. (2021) 40:50–8. doi: 10.12938/bmfh.2020-046
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fnut.2021.745609/full


Tidak ada komentar: